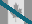Z-Phe-Arg-AMC
Fluorogenic cathepsin substrate / Z-Phe-Arg-AMC (70382-26-2) is a fluorogenic substrate1 for the following cathepsins (kcat/Km in M-1s-1): B (105)2, C/DPP-I (104)3, F (106)4, K/O2 (105)3, L (106)3, L2/V (105)5, O6, S (104)7, X/Z (104)8. The peptide is also cleaved by plasma kallikrein and kallikrein 89, and papain (kcat/Km=105 M-1s-1).3 Excitation: 365nm, Emission: 440nm.5
Biochemicals & reagents
70382-26-2
Z-FR-AMC hydrochloride; Cbz-Phe-Arg-AMC hydrochloride
1 Tavares et al. (2004), Design of potent, selective, and orally bioavailable inhibitors of cysteine protease cathepsin k; J. Med. Chem., 47 588 2 Therrien et al. (2001), Cathepsins X and B can be differentiated through their respective mono- and dipeptidyl carboxypeptidase activities; Biochemistry, 40 2702 3 Nägler et al. (1999), Interdependency of sequence and positional specificities for cysteine proteases of the papain family; Biochemistry, 38 4868 4 Wang et al. (1998), Human cathepsin F. Molecular cloning, functional expression, tissue localization, and enzymatic characterization; J. Biol. Chem., 273 32000 5 Brömme et al. (1999), Human cathepsin V functional expression, tissue distribution, electrostatic surface potential, enzymatic characterization and chromosomal localization; Biochemistry, 38 2377 6 Velasco et al. (1994), Human cathepsin O. Molecular cloning from a breast carcinoma, production of the active enzyme in Escherichia coli, and expression analysis in in human tissues; J. Biol. Chem., 269 27136 7 Kopitar et al. (1996), Folding and activation of human procathepsin S from inclusion bodies produced in Escherichia coli; Eur. J. Biochem., 236 558 8 Klemenčič et al. (2000), Biochemical characterization of human cathepsin X revealed that the enzyme is an exopeptidase, acting as carboxymonopeptidase or carboxydipeptidase; Eur. J. Biochem., 267 5404 9 Kishi et al. (2006), Activation and enzymatic characterization of recombinant human kallikrein 8; Biol. Chem., 387 723
-20°C (protect from light)
TARGET: Substrate / Probe; Protease